Specific 18 F-FDHT Accumulation in Human Prostate Cancer Xenograft Murine Models Is Facilitated by Prebinding to Sex Hormone–Binding Globulin
Benjamin M. Larimer1, Frank Dubois1, Emily Bloch1, Sarah Nesti1, Michael Placzek1, Giorgia Zadra2,3, Jacob M. Hooker1, Massimo Loda2,3 and Umar Mahmood1
2018 October
ABSTRACT
Tremendous efforts are currently dedicated to the development of novel therapies targeting the androgen receptor (AR), the major driver of prostate cancer disease and its progression to castration resistance. The ability to noninvasively interrogate AR expression over time in murine models of prostate cancer would permit longitudinal preclinical analysis of novel compounds that could not otherwise be accomplished ex vivo. Although PET imaging with 16β-18F-fluoro-5α-dihydrotestosterone (18F-FDHT) has successfully quantified AR levels clinically, no rodent model of 18F-FDHT imaging has been reported so far. One difference between humans and rodents is the absence in the latter of the sex hormone–binding globulin (SHBG), a glycoprotein that binds to testosterone in the bloodstream, Here, we explore the role of SHBG in developing a working model of rodent AR imaging. Methods: Three human prostate cancer cell lines and xenografts (LNCaP, 22Rv1, and PC3) were used to examine the uptake of free 18F-FDHT and SHBG-bound 18F-FDHT. Both ligands were examined for stability and competitive binding to AR over time in vitro before in vivo studies. PET/CT imaging was used to dynamically measure the uptake of both tracers over 4 h, whereas specificity was determined by competitive binding with the AR antagonist enzalutamide. Results:AR levels correlated with the uptake of both 18F-FDHT and SHBG-18F-FDHT in prostate cancer cell lines. Interestingly, whereas both free and SHBG-bound 18F-FDHT had a similar cellular accumulation at 1 and 2.5 h, SHBG-18F-FDHT accumulated at significantly higher levels after 4 h—evidence that receptor-mediated uptake of SHBG accounted for later time-point differences. This observation was also seen in 22Rv1 tumor–bearing mice, in which SHBG-18F-FDHT exhibited a significantly increased uptake (average tumor-to-background ratio [TBR], 1.62 ± 0.62) in comparison to unbound 18F-FDHT (TBR, 0.81 ± 0.08) at 4 h. Furthermore, the specificity of the SHBG-18F-FDHT accumulation at 4 h was demonstrated by a reduced tumor uptake after AR blockade with enzalutamide (TBR, 1.07 ± 0.13). Conclusion: Prebinding of 18F-FDHT to SHBG allows accurate and quantitative PET imaging of AR levels in murine models of prostate cancer. This procedure may permit the use of PET imaging to study the longitudinal effects of AR-targeting therapies, accelerating novel-drug development.
KEYWORDS
Prostate cancer is the second most common cause of cancer-related death and the cancer with the highest incidence in men. As such, prostate cancer is the focus of many novel therapeutics, including those that target the androgen receptor (AR) (1). Initially, most prostate cancers show a response to treatment by androgen deprivation because androgens are the physiologic growth factor for prostate cells (2). However, over time, almost all prostate cancers become unresponsive to treatment (3). These castration-resistant prostate cancers are difficult to manage and often lethal. The malignancy can develop through several mechanisms, but ultimately all lead to increased intracellular AR signaling (4,5). Despite the clinical relevance of AR quantification, histopathologic examinations of biopsy samples may be unable to provide an accurate characterization. In fact, clinical examination has demonstrated that AR expression levels can differ widely between metastatic sites in a single patient (6). Thus, the limited sampling of a biopsy is unable to capture the heterogeneity of AR expression across primary and metastatic sites of prostate cancer, making accurate quantification of AR levels difficult to obtain (7).
To overcome the limitations of biopsy, noninvasive imaging of AR can be used to quantify expression in both primary tumors and metastatic sites simultaneously, allowing for subsequent evaluation and guidance of AR-targeted therapies. PET offers a quantitative imaging technique that has been used extensively for both cancer detection and characterization in humans. Furthermore, an effective PET tracer for AR, 16β-18F-fluoro-5α-dihydrotestosterone (18F-FDHT), has already been used clinically to permit detection and relative quantification of AR expression noninvasively (8). 18F-FDHT has been demonstrated to identify AR-positive lesions with high sensitivity and specificity in patients and to detect changes in AR over the course of therapy, suggesting that 18F-FDHT is a promising candidate for use in monitoring AR clinically (9).
Although 18F-FDHT PET imaging has been successful clinically, paradoxically, a mechanistic understanding in rodent tumor models has been lacking. This lack could be attributed to the absence of sex hormone–binding globulin (SHBG) expression in most mature rodents, including mice, and previous 18F-FDHT rodent studies support this hypothesis (10). A direct comparison of biodistribution was performed between immature rodents, which express a molecule similar to SHBG termed androgen-binding protein, and mature rats, which lack any FDHT-binding proteins (10). It was found that mature rodents had significant levels of accumulation in bone, presumably through defluorination of 18F-FDHT, whereas immature rats did not, indicating a protective role of androgen-binding protein for 18F-FDHT metabolism. Additionally, other species (e.g., nonhuman primates and rabbits) successfully imaged with 18F-FDHT have serum SHBG or androgen-binding protein, but mice express no SHBG or analogs (11,12). The lack of positive results for 18F-FDHT imaging in various rodents thus far led us to investigate whether 18F-FDHT imaging can be improved in the mouse models of human prostate cancer most commonly used to assess novel androgen-modulating therapies. We brought about this improvement by binding 18F-FDHT to SHBG and systematically studying the properties of the combination. A method to quantitatively monitor AR expression noninvasively in preclinical models of prostate cancer would permit repeated, global quantification of the receptor. This, in turn, could allow monitoring of the kinetics and outcomes of AR-modulating therapeutics.
In this study, we sought to accurately determine the effects of SHBG on 18F-FDHT kinetics and biodistribution in mouse models of human prostate cancer. Specifically, we investigated whether it is possible to accurately quantify relative AR expression levels in mouse models of prostate cancer using 18F-FDHT–based PET imaging and whether specific tumor uptake in these models can be improved using SHBG-bound 18F-FDHT.
MATERIALS AND METHODS
Materials and Cell Lines
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich. LNCaP (CRL-1740), PC3 (CRL-1435), and 22Rv1 (CRL-2505) human prostate cells were purchased from ATCC and cultured in RPMI-1640 medium (ThermoFisher) supplemented with 10% fetal bovine serum (Aleken).
Murine Models
The mice were housed and maintained by the Center for Comparative Medicine following animal protocols approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee. Male nu/nu mice were purchased from Charles River Laboratories. For tumor inoculation, approximately 1 × 106 cells were mixed in a 1:1 (v:v) ratio with Matrigel (Corning) and subcutaneously injected into the upper right flank of nu/nu mice. 22Rv1 xenograft models were injected in orchiectomized mice to simulate clinical castration conditions, whereas LNCaP xenografts, which require androgens for growth, were generated in intact mice.
18F-FDHT Synthesis, Purification, and SHBG Interaction
18F-FDHT was synthesized by modifying a procedure previously described in the literature (13). Crude 18F-FDHT was purified by reversed-phase high-pressure liquid chromatography using a 9.4 × 250 mm Eclipse XDB-C18 (5 μm) column (Agilent) and eluted with 40% acetonitrile/60% 50 mM KH2PO4 at 4 mL/min. Nonradioactive peaks were analyzed by ultraviolet light (210 nm). Collected 18F-FDHT was diluted with 20 mL of water and captured on a C18 light Sep-Pak cartridge (Waters) preconditioned with 1 mL of ethanol and 4 mL of water. 18F-FDHT bound to the cartridge was washed with 5 mL of water and then eluted with ethanol and diluted with sterile 0.9% saline solution for a less than 10% final concentration of ethanol. Product purity and identity (coinjected with 18F-FDHT) were confirmed by analytic high-pressure liquid chromatography using an Agilent Eclipse Plus-C18 (5 μm) eluting with 40% acetonitrile/60% 50 mM KH2PO4 at 2 mL/min (ultraviolet, 215 nm), with a retention time of 6.1 min. For experiments involving SHBG (Abcam)-bound 18F-FDHT, 9 μg of purified recombinant human SHBG per mouse (54–108 mg of total protein) were incubated at 37°C for 30 min with approximately 740 MBq of 18F-FDHT. Size-exclusion chromatography using a 10-kDa cartridge (GE Healthcare) was performed to eliminate free 18F-FDHT. The final SHBG-18F-FDHT complex was quantified by dose calibrator.
In Vitro Characterization of Free and SHBG-Bound 18F-FDHT
Uptake of free 18F-FDHT in LNCaP, 22Rv1, and PC3 prostate cancer cells was probed by incubating the purified radiotracer in 100 μL of serum-free medium with cells grown to 70% confluency in a 96-well tissue culture plate (Corning). Additional specificity for the AR was also determined by incubating separate wells of LNCaP cells with 10-fold dilutions of the AR antagonist enzalutamide (range, 0.1–100 μM) (Selekchem). After incubation with the probe, the cells were washed 3 times with 100 μL of Dulbecco phosphate-buffered saline (ThermoFisher) and lysed in 100 μL of 1% sodium dodecyl sulfate. Recovered cell lysate was assayed for radioactivity by a 2480 Wizard2automatic γ-counter (Perkin Elmer), and total protein was determined using the Pierce BCA Protein Assay Kit (Life Technologies) for standardization to total protein per well.
Competitive binding of enzalutamide to SHBG in the presence of 18F-FDHT was assessed by the addition of 0.1–100 μM enzalutamide to SHBG before the addition of 0.37 MBq of 18F-FDHT. The stability of both 18F-FDHT and SHBG-18F-FDHT was assessed by incubating the radiotracers with 100 μL of either phosphate-buffered saline, fetal bovine serum, or human serum for 1 h followed by cell-binding analysis as described previously. The specificity of SHBG-18F-FDHT in comparison to 18F-FDHT was analyzed by incubation of LNCaP cells grown to 70% confluency for 1 h with 0.37 MBq of the designated radiotracer in 100 μL of serum-free medium in the presence or absence of 100 μM enzalutamide, followed by quantification of cell lysate radioactivity and total protein. Finally, an examination of the temporal kinetics of cell binding of 0.37 MBq of either 18F-FDHT or SHBG-18F-FDHT was performed at 1, 2, and 4.5 h of incubation in LNCaP cells before lysis and quantification of total protein and radioactivity, in the same manner as described previously.
PET Imaging
Comparison of 18F-FDHT and SHBG-18F-FDHT uptake analysis was performed on nu/nu mice bearing LNCaP tumors and nu/nu orchiectomized mice bearing 22Rv1 tumors. For 18F-FDHT PET imaging experiments, 4 nu/nu mice bearing LNCaP tumors and 4 orchiectomized nu/nu mice bearing 22Rv1 tumors were used. For SHBG-18F-FDHT PET imaging, 4 LNCaP and 8 22RV1 tumor–bearing mice were used, with the 22Rv1 tumor–bearing mice being divided randomly into 2 equal groups receiving either vehicle or enzalutamide (20 mg/kg) 12 h and 1 h before injection. 18F-FDHT and SHBG-18F-FDHT were prepared at 37–179 MBq/mL (3–16 ng of FDHT by mass) in normal saline and injected intravenously. PET/CT images were acquired on a Triumph small-animal PET/CT device (GE Healthcare) for 15 min at a single bed position at 1, 2.5, or 4 h after injection. Images were reconstructed by 3-dimensional ordered-subsets expectation maximization using 4 subsets and 15 iterations. Tumor and heart (as blood pool background) PET signal was quantified by drawing a 3-dimensional region of interest using CT anatomic guidance on VivoQuant software (version 2.5; InviCRO).
Statistical Analysis
Statistical analysis and graphing were performed using Prism software (version 6; GraphPad Software). Differences in cell uptake were compared using 1-way ANOVA. Differences in 18F-FDHT and SHBG-18F-FDHT competitive inhibition in vitro were analyzed using a Student t test. Differences in tumor uptake were compared using 2-way ANOVA.
RESULTS
18F-FDHT Synthesis, Purification, and Analysis
18F-FDHT was synthesized using a modification of a previously described procedure (13). Nonradioactive peaks were analyzed by ultraviolet absorbance (210 nM) with an approximately 19-min retention time of 18F-FDHT (retention factor, 7.63) (Fig. 1A). The collected final purified product afforded an injectable solution of 18F-FDHT with an average radiochemical yield of 40% ± 12% (end of synthesis, decay-corrected) and specific activity of 340 ± 55 GBq/μmol (end of synthesis). The purity of the final formulated product was more than 99%, and the retention time matched the FDHT standard (6.1 min). After confirmation of identity and radiochemical purity, we tested 18F-FDHT for specificity and affinity to the AR using cell-binding assays. In highly AR-expressing LNCaP cells, 18F-FDHT uptake was measured at 3.05 ± 0.49 × 105 counts per minute (CPM)/mg of protein. The moderately AR-expressing 22Rv1 cell line 22RV1 accumulated significantly lower levels of tracer, at 6.16 ± 0.21 × 104 (CPM)/mg of protein, and the AR-negative PC3 cells showed nonspecific background activity of 1.95 ± 0.50 × 104 CPM/mg (Fig. 1B). The AR antagonist enzalutamide demonstrated a dose-dependent blockade of binding. Furthermore, enzalutamide was able to decrease 18F-FDHT binding by 82% in LNCaP cells at a concentration of 100 μM, a level comparable to the nonspecific background activity measured in PC3 cells (Fig. 1C). Taken together, these data confirmed that the synthesized compound was biologically active for in vivo analysis.
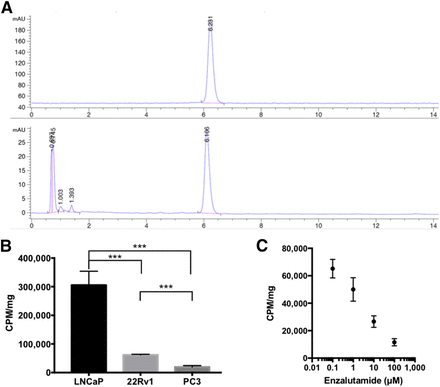
FIGURE 1.
(A) High-pressure liquid chromatography radiographic (top) and ultraviolet (bottom) traces after 18F-FDHT synthesis. 18F-FDHT peaks detected by both ultraviolet (210 nM) and radioactive traces were consistent with successful synthesis of target molecule. (B) 18F-FDHT uptake measured in high-AR (LNCaP), moderate-AR (22Rv1), and low-AR (PC3) cells. Bars represent mean of 6 replicates, with error bars denoting SEM. (C) Competitive binding of enzalutamide to LNCaP cells, demonstrating dose-dependent decrease of 18F-FDHT uptake with increasing doses of enzalutamide. Dots represent mean of 6 samples quantified by γ-counter and measured in CPM and normalized to total protein, with error bars denoting SEM. ***P < 0.001.
In Vivo Analysis of 18F-FDHT
To test 18F-FDHT in a variety of biologic settings, 2 models, androgen-sensitive LNCaP tumors and castration-resistant 22Rv1 tumors, were chosen for analysis. The 22Rv1 tumors were investigated in the setting of orchiectomy, since their growth is independent of androgen signaling. Despite the use of 2 models with marked differences in AR expression with and without androgen deprivation, low tracer accumulation was observed across all groups. Specific tumor uptake, as measured by a ratio of mean tumor intensity divided by mean left ventricle intensity (tumor-to-background ratio [TBR]) was below 1 in all groups when measured at 1 h after injection (Fig. 2A). Intact LNCaP mice had an average TBR of 0.84 ± 0.12, and orchiectomized 22Rv1 tumor–bearing mice had an average TBR of 0.81 ± 0.08 (Fig. 2B). Visualization of tracer accumulation was consistent with previous murine examinations of 18F-FDHT, with hepatobiliary, bowel, and bone accumulation evident at 1 h after injection.
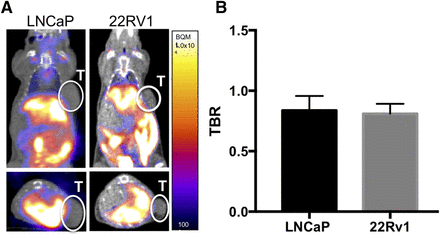
FIGURE 2.
(A) Coronal (top) and axial (bottom) images of mice bearing LNCaP and 22Rv1 human prostate cancer xenografts 1 h after injection with 18F-FDHT. Signal accumulation is consistent with previously observed hepatobiliary clearance of tracer, with no accumulation visualized in either tumor. (B) Tumor uptake normalized for injection by comparison to left ventricle uptake in LNCaP and 22Rv1 mice. Bars represent mean of 4 replicates, with error bars representing SEM. T = tumor.
In Vitro Comparison of 18F-FDHT and SHBG-18F-FDHT
SHBG was bound to 18F-FDHT, and the combined molecule was compared with 18F-FDHT alone across several in vitro analyses. The stability and functionality of both 18F-FDHT and SHBG-18F-FDHT under different serum conditions were assessed, measuring cell-bound activity as an output. The binding of 18F-FDHT did not differ from that of SHBG-18F-FDHT in phosphate-buffered saline (5.12 ± 2.15 × 105 vs. 3.40 ± 0.28 × 105 CPM/mg, respectively), bovine serum (4.48 ± 0.94 × 105 vs. 3.37 ± 0.56 × 105CPM/mg, respectively), or mouse serum (3.75 ± 0.74 × 105 vs. 3.11 ± 0.75 × 105CPM/mg, respectively) (Fig. 3A). To test the specificity of both tracers, competitive binding experiments with the AR antagonist enzalutamide were used. Specific binding and nonspecific binding were similar for both tracers at 1 h (unblocked: 18F-FDHT = 5.04 ± 0.50 × 105 CPM/mg, SHBG-18F-FDHT = 5.50 ± 0.93 × 105 CPM/mg; blocked/unspecific: 18F-FDHT = 1.19 ± 0.52 × 105 CPM/mg, SHBG-18F-FDHT = 1.27 ± 0.38 × 105 CPM/mg), with similar percentages of blocking achieved, at 76% and 77%, for 18F-FDHT and SHBG-18F-FDHT, respectively. Enzalutamide was also assayed for competitive binding with 18F-FDHT for SHBG. Minimal 18F-FDHT displacement occurred across a range of concentrations, and even at concentrations as high as 100 μM, up to 57% of the 18F-FDHT remained bound, indicating that enzalutamide did not affect the SHBG-18F-FDHT complex. Finally, the kinetics of both 18F-FDHT and SHBG-18F-FDHT were assayed over 4 h. Although there was similar uptake at both 1 and 2.5 h for free 18F-FDHT (1 h, 5.50 ± 0.46 × 105 CPM/mg; 2.5 h, 2.03 ± 0.19 × 105 CPM/mg) and bound 18F-FDHT (1 h, 5.04 ± 0.35 × 105 CPM/mg; 2.5 h, 2.40 ± 0.34 × 105CPM/mg), at 4 h a significant increase occurred for the SHBG-bound 18F-FDHT (4.32 ± 0.14 × 105 CPM/mg of 18F-FDHT vs. 1.04 ± 0.06 × 106 CPM/mg of SHBG-18F-FDHT, P< 0.0001), as shown in Figure 3D. These results provide evidence that SHBG decisively changes the kinetics of specific 18F-FDHT binding.
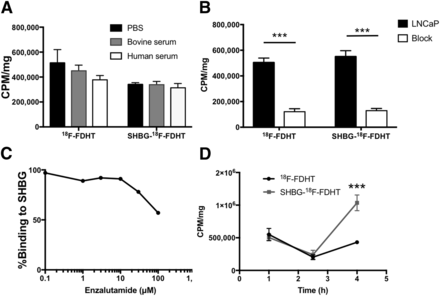
FIGURE 3.
(A) Uptake of 18F-FDHT and SHBG-18F-FDHT in LNCaP cells after 1 h of incubation in phosphate-buffered saline (PBS), bovine serum, or human serum, standardized by protein per well. Bars represent mean of 6 replicates, and error is denoted by SEM. (B) Competitive binding analysis of18F-FDHT and SHBG-18F-FDHT with AR antagonist enzalutamide. Each bar is mean of 6 replicates ± SEM. (C) Competitive binding analysis of 18F-FDHT to SHBG with AR antagonist enzalutamide, demonstrating minimal competition between 18F-FDHT and enzalutamide for binding to SHBG. (D) Time course of 18F-FDHT and SHBG-18F-FDHT binding to LNCaP cells. Although similar levels of binding are observed at 1 and 2.5 h, significant increase in uptake is seen at 4 h in only cells incubated with SHBG-18F-FDHT. Dots represent mean of 6 replicates ± SEM. ***P < 0.001.
In Vivo Comparison of 18F-FDHT and SHBG-18F-FDHT
Purified 18F-FDHT was bound to SHBG before injection in a similar manner to in vitro experiments, and uptake was quantified over time in intact LNCaP and orchiectomized 22Rv1 tumor–bearing mice. The 22Rv1 mice were randomly distributed into vehicle and enzalutamide-treated groups to test for specificity. At 1 h after injection, the TBRs of LNCaP (0.72 ± 0.12), 22Rv1 (0.94 ± 0.14), and enzalutamide-blocked 22Rv1 tumors (0.74 ± 0.06) for SHBG-bound 18F-FDHT were all statistically similar. The TBRs for both LNCaP and 22Rv1 tumors were also similar between free and SHBG-bound 18F-FDHT. The similarity between the 2 groups existed despite a significantly lower blood signal in the free 18F-FDHT group (Fig. 4C), indicating that although there are differences in clearance of the 2 tracers at 1 h, the tumor accumulation at 1 h does not appear to be specific for any group. Given the evidence that free 18F-FDHT was significantly more metabolized at 1 h, only SHBG-bound 18F-FDHT was analyzed at later time points (Fig. 4). For SHBG-18F-FDHT, TBRs increased in both blocked and unblocked 22Rv1 tumors from 1 to 2.5 h, with TBR levels increasing to 1.00 ± 0.19 in 22Rv1 and 1.21 ± 0.02 in enzalutamide-blocked 22Rv1 tumors. No increase was observed at 2.5 h in the intact LNCaP prostate cancer model. At 2.5 h after injection, a significant difference in TBR was observed between the 22Rv1 and LNCaP models. At 4 h after injection, a statistically significant divergence in TBR was observed between blocked and unblocked AR in 22Rv1 tumors. Unblocked 22Rv1 tumors demonstrated the highest TBR, at 1.62 ± 0.26, followed by enzalutamide-blocked 22Rv1 tumors, at 1.07 ± 0.13, and finally LNCaP tumors, at 0.56 ± 0.19. These differences were driven by a significant increase in the TBR of 22Rv1 tumors, which was not observed in either the AR-blocked or the intact LNCaP tumor models. This finding confirmed the observed in vitro data, indicating SHBG-mediated AR-specific 18F-FDHT uptake in prostate cancer xenografts at 4 h.
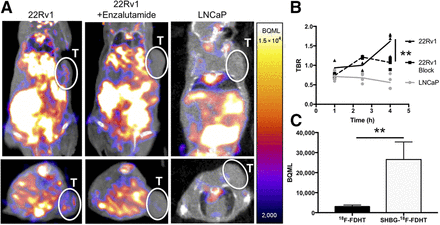
FIGURE 4.
(A) Coronal (top) and axial (bottom) images of 22Rv1, 22Rv1-plus-enzalutamide, and LNCaP tumor–bearing mice 4 h after injection with SHBG-18F-FDHT. Hepatobiliary clearance is similar to that of 18F-FDHT; however, accumulation is also visualized in 18F-FDHT-SHBG–injected 22Rv1 mice. (B) Time course quantification of tumor accumulation standardized to injection as TBR. Each dot represents individual mouse from 22Rv1, 22Rv1 block, and LNCaP groups. (C) Blood levels quantified by PET for 18F-FDHT and SHBG-18F-FDHT. **P < 0.01. T = tumor.
IPI-9119 induces ER stress response and protein translation inhibition. (A) Immunoblotting showing the induction of the ER stress marker p-eiF2α in LNCaP and LNCaP-95 cells treated with IPI-9119 (IPI) for 3 and 6 d. (B) SUnSET assay using lysates from LNCaP and LNCaP-95 cells treated with IPI for 3 and 6 d. (C) Representative immunoblotting showing the down-regulation of translation initiaton cofactor eiF4B, following treatment with IPI for 6 d. Experiment was repeated twice (LNCaP) and four times (LNCaP-95). (D) Immunoblotting showing the rescue of AR expression in LNCaP, following reduction of ER stress using the PERK inhibitor GSK2606414 (100 nM, 24 h; 1-h pretreatment). Phosphorylation of PERK is shown as an upward shift of the protein molecular weight. (E) Immunoblotting showing the rescue of AR expression in LNCaP, following reduction of ER stress using the Ca2+ chelant bapta (10, 20 μM, 12 h; 1-h pretreatment). Next to the immunoblotting, densitometric analysis of p-eiF2a is shown. Values are reported as normalized arbitrary units (AU). (F) Representative immunoblotting showing concomitant rescue of IPI-mediated AR/AR-V7 reduction and ER stress, following incubation with palmitate for 3 d. Palmitate (50 μM) was complexed with BSA (molar ratio 6:1), while control cells were treated with BSA only. (G) Schematic representation of the involvement of ER stress in mediating IPI effects.
DISCUSSION
The work herein demonstrates a novel method to quantify AR expression differences in mouse models of prostate cancer with PET imaging by using 18F-FDHT bound to SHBG and imaging at prolonged time points. Our work confirmed that although 18F-FDHT alone is capable of specifically binding the AR in vitro, there is no specific uptake of 18F-FDHT in human prostate cancer xenografts in mice. Previous studies have investigated accumulation in mature and immature rodent prostate and models of human ovarian cancer and glioma, but thus far prostate cancer had not been analyzed (10,14). Although each model has demonstrated AR expression, the levels of AR expression in prostate cancer are more clinically relevant, since most AR-targeted therapies aim to manage both castration-sensitive and refractory prostate cancer (15–17). Thus, successful 18F-FDHT imaging in a murine model of prostate cancer might not only aid development of these AR-targeted therapies but also contribute to the knowledge of 18F-FDHT accumulation that is mediated by SHBG. We hypothesized that a more systematic examination of the role of SHBG in 18F-FDHT rodent animal models could facilitate successful imaging.
Although SHBG is best known for its ability to modulate the levels of free steroids in the blood, the protein is also capable of binding cells and initiating signaling in cancer cells, including prostate cancer (18,19). 18F-FDHT has been studied extensively both in vitro and in vivo, but no data have been reported in vitro when bound to SHBG. Conversely, a large body of work centered around the free-hormone hypothesis has investigated various facets of SHBG and dihydrotestosterone cell binding and their respective internalization and signaling. Our data agree with the work by Hammes et al., demonstrating uptake of both SHBG and dihydrotestosterone by rat choriocarcinoma cells (20), which was postulated to occur through megalin-induced endocytosis. Our work expanded on this analysis to include uptake over time and a direct comparison of the uptake of free 18F-FDHT and SHBG-bound 18F-FDHT. Because the dissociation half-life of dihydrotestosterone from SHBG is on the order of 43 s (21), early-time-point binding may reflect more heavily on the dissociated 18F-FDHT, which would explain similar uptake at early time points. The use of a competitive antagonist also supports this hypothesis, as most of the cell-associated signal is blocked by the addition of enzalutamide. We hypothesize that differences in accumulation at later time points occur, at least in part, because of the biology of SHBG internalization. Free SHBG has been demonstrated to bind megalin and to be endocytosed, resulting in accumulating cytoplasmic levels of the protein (22). Once internalized, SHBG is able to retard the efflux of 18F-FDHT (20), providing an additional mechanism of enhanced 18F-FDHT retention and therefore a larger pool of tracer capable of binding the AR. Given the slow association of SHBG to cellular membranes (23), it is likely that only at the 4-h time point is accumulation driven by this protective or reservoir effect (24).
The observation that SHBG-bound 18F-FDHT potentially had different binding kinetics from free 18F-FDHT in vitro provided a significant impetus to continue the examination in vivo. The similar results observed in vivo also supported the hypothesis that SHBG plays multiple roles in specific 18F-FDHT delivery to the tumor. The significant decrease in measured blood radioactivity for unbound 18F-FDHT in comparison to bound 18F-FDHT signals an increased stability that would be expected of a steroid carrier. Additionally, the dissociation rate of 43 s would provide sufficient time for the SHBG-18F-FDHT complex to be delivered to the tumor. In the tumor microenvironment, the enhanced permeability and retention effect probably also contributes to indistinguishable differences at early time points, which can be clarified over time as SHBG diffuses out of the tumor microenvironment. One limitation of this work is that 18F-FDHT that has dissociated from SHBG after injection cannot be discerned from SHBG-bound tracer, but this limitation does not rule out other potential significant interactions with proteins such as albumin.
In addition to the exploration of SHBG-18F-FDHT kinetics, examination of biologically distinct human tumor xenografts provided additional considerations for successful rodent imaging. The universally low 18F-FDHT accumulation, whether SHBG-bound or free, in LNCaP xenografts emphasizes that the presence of endogenous sex hormones makes a significant difference in murine models. Although the most likely explanation for the significantly lower accumulation is direct competition of endogenous hormones with 18F-FDHT, other, yet-unknown, effects of orchiectomy could contribute to increased accumulation in 22Rv1 tumors.
CONCLUSION
The role of the AR in prostate cancer development, recurrence, and morbidity has been well defined. As such, numerous therapies that target the AR are under development and currently in clinical trials. Although large-scale preclinical ex vivo examinations of the effects of any drug on AR expression or tumor growth can be accomplished ex vivo, the ability to monitor both simultaneously and repeatedly over time to follow the individual response to therapy cannot be accomplished without molecular imaging. By prebinding 18F-FDHT with SHBG, we showed the possibility of specifically imaging ARs in human prostate cancer xenograft mouse models. This procedure might permit novel therapeutic strategies targeting AR and deepen our understanding of the kinetics of AR receptor signaling.
DISCLOSURE
This work was supported by DOD synergistic award W81XWH-14-1-0406 to Umar Mahmood and Massimo Loda. No other potential conflict of interest relevant to this article was reported.
FOOTNOTES
Published online May 31, 2018.
© 2018 by the Society of Nuclear Medicine and Molecular Imaging.