Research
The overarching goal of my research is to use molecular imaging to help stratify cancer patients for therapy selection and subsequently monitor response to therapy. One of the major challenges in cancer therapy today is identifying which patients would be the best candidates for specific therapies, and after they have initiated therapeutic intervention, quickly and accurately analyzing response to these therapies. While traditional approaches to patient stratification and therapeutic monitoring hold considerable utility, molecular imaging provides a unique insight into the in vivo processes that can augment the current suite of clinical assessments.
In order to develop and characterize novel molecular imaging agents and approaches, our research focuses in two areas. The first area is the development of novel molecular imaging probes, specifically focused on PET imaging, that can provide rapid and quantitative analysis of biological processes related to cancer therapy. Through the use of a technology termed bacteriophage (phage) display, we have the capability to develop specifically targeted biological probes against virtually any target, allowing for the development of novel diagnostic assays against high value targets that can provide insight into areas such as therapeutic target expression and mechanisms of resistance. The second area of focus is the combination of PET imaging and molecular biology to provide unique insight into cancer tumor biology that is not possible using standard research tools. Non-invasive imaging allows for quantification of targets longitudinally, which permits an understanding of how expression of a protein at an earlier timepoint may affect outcomes at later timepoints. Novel biomarkers of response, potential therapeutic targets and insights into disease progression can be gleaned from this work, providing opportunities for future endeavors. To highlight these efforts in greater detail, the following sections expand upon both areas of interest.
68Ga-NOTA-HER3-1 PET Imaging and Tumor HER3 Expression Correlation of a peptide that was generated through phage display and is currently being filed as an Investigational New Drug with the FDA
Novel Imaging Probe Development
While there are many approaches to non-invasive imaging that hold tremendous value, positron emission tomography (PET) imaging is one that we find very appealing. The ability to interrogate specific proteins of interest can inform on the exact location, expression level, and dynamic changes of virtually any target. A unique consideration for developing in vivo targeting agents, especially for cancer, is that biodistribution and pharmacokinetics of any individual tracer can greatly affect the accuracy and extent of applications for which it can be used. Traditionally, antibodies are used to specifically detect proteins of interest. This is with good reason, as antibodies bind specifically and with high affinity to their target of interest. Unfortunately, for in vivo PET imaging, antibodies were designed to circulate in the blood stream for long periods of time, generating significant background signal that makes detecting fine biochemical perturbations difficult. In order to overcome this, my research focuses on designing much smaller peptides and single domain nanobodies as imaging agents. Because, unlike antibodies, these cannot be generated simply by immunizing a rabbit or mouse, my research employs a combinatorial chemistry technique termed phage display to select ligands of interest. This technique has tremendously shaped the field of protein evolution, and the Nobel prize in 2018 was awarded to George P Smith in part for his discovery of phage display. We have utilized phage display to design peptide imaging agents targeting cancer antigens including HER2, HER3 and EGFL6. These agents have been used to detect and quantify breast and prostate cancer expression and diagnose resistance to targeted therapies.
In addition to peptides, we have also designed a novel library that displays single domain antibodies that are typically found in the blood of dromedaries including camels, llamas and alpacas. we have engineered these antibodies to be humanized so that they will not elicit an immune response in humans. The synthetic library provides an antibody library that is 10 times more diverse than theoretical calculations of the human antibody diversity. Because it is displayed using phage display, mock immunizations can be carried out in the lab in a much more rapid manner and with many different targets screened simultaneously. Although this development is in its early stages, we have generated promising data for targets including CD8, CD3 and CD39 which may help to understand the role of T cells and resistance mechanisms to immunotherapy.
Implementation
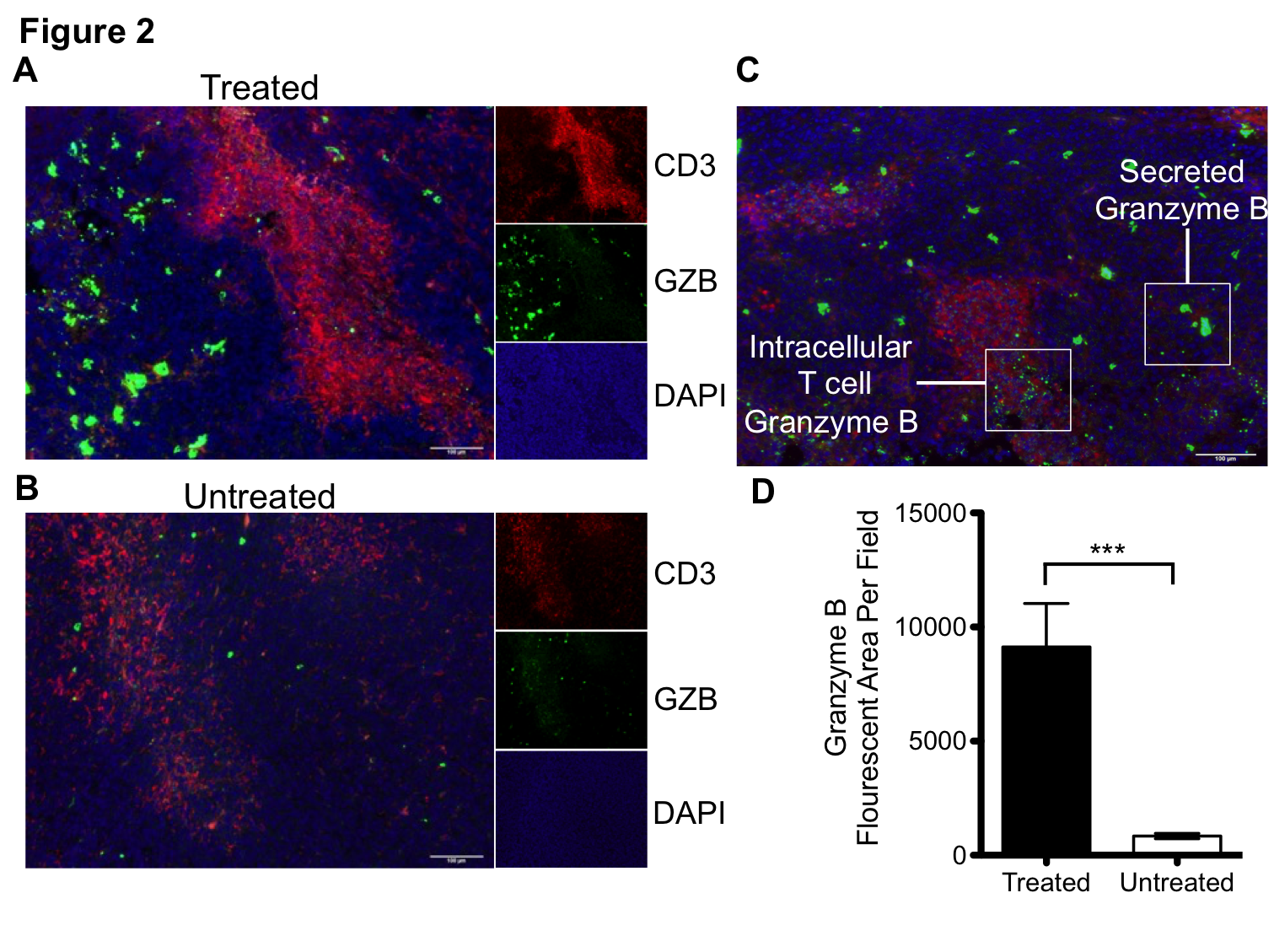
The concept of using PET imaging to molecularly characterize cancer is still an emerging one. Although there are many examples of molecularly targeted PET imaging agents, biopsy and blood tests are still the primary tools an oncologist uses to characterize cancer. This is with good reason, as these tools have the power to assay thousands of individual proteins. However, one thing they lack is the ability to interrogate processes as they occur throughout the entire body, and with a level of precision and quantification that permits diagnosis. For instance, in the field of immuno-oncology, while a biopsy can determine the level of PD-L1 in a single tumor at a single time point, it cannot predict response. This is a major problem, as most patients treated with immunotherapy will derive no benefit from it. We have developed a molecularly targeted imaging agent against granzyme B that can predict response to immunotherapy shortly after therapy initiation, prior to anatomic changes in the tumor. Not only does this peptide have tremendous potential as a clinical diagnostic tool, but it also offers a unique window into tumor response. By performing granzyme B PET imaging, we can effectively see into the future to label tumors as responsive or non-responsive. From there, we can interrogate the phenotype of responders versus non-responders at a time point where all other measures are thus far ineffective. This is a single example of using non-invasive imaging to understand biology, but the concept can be applied to a whole host of other targets and diseases.
Clinical Translation
The goal of our work is to create agents which can personalize cancer treatment. As such, discoveries made in our lab are prioritized and pushed towards clinical translation. Working with the world-class radiopharmacy at UAB, we are pushing towards our first first-in-human study of an agent developed by our group. The imaging agent, which targets granzyme B, is going to be tested in patients with melanoma and non-small cell lung cancer in order to assess granzyme B levels following immunotherapy.
The rapid translation from bench to bedside is the paradigm from which we operate. Our goal is to discover and develop novel targeting agents and rapidly pursue clinical applications. The environment at UAB is highly conducive to clinical translation, and we aim to take full advantages of the resources available.
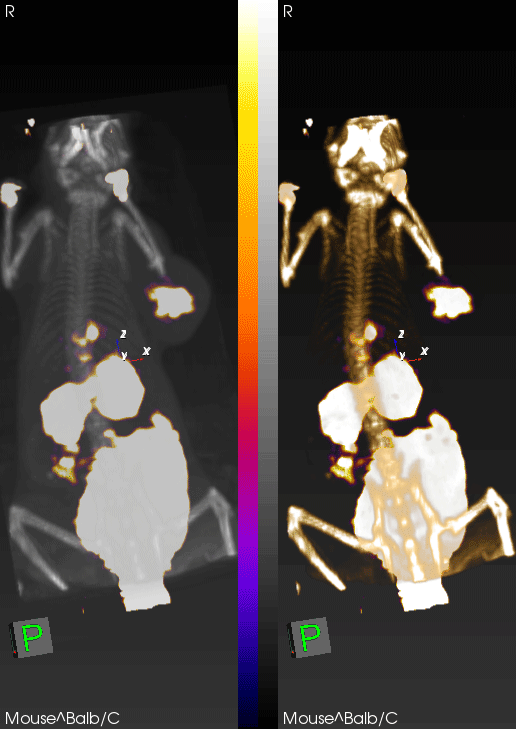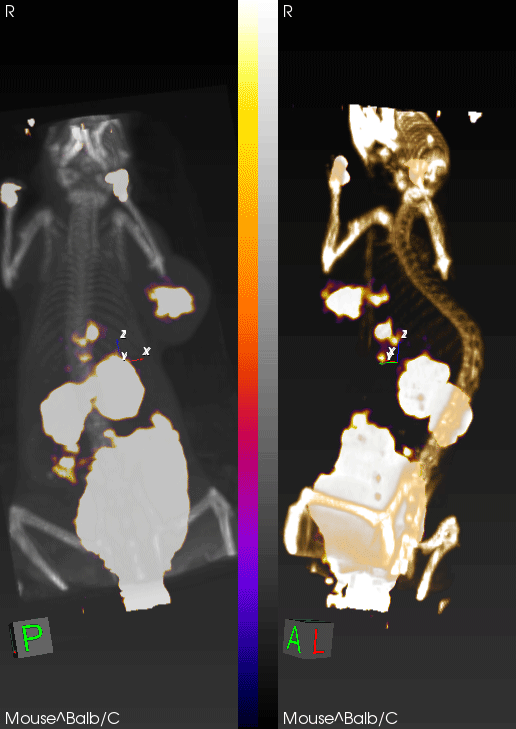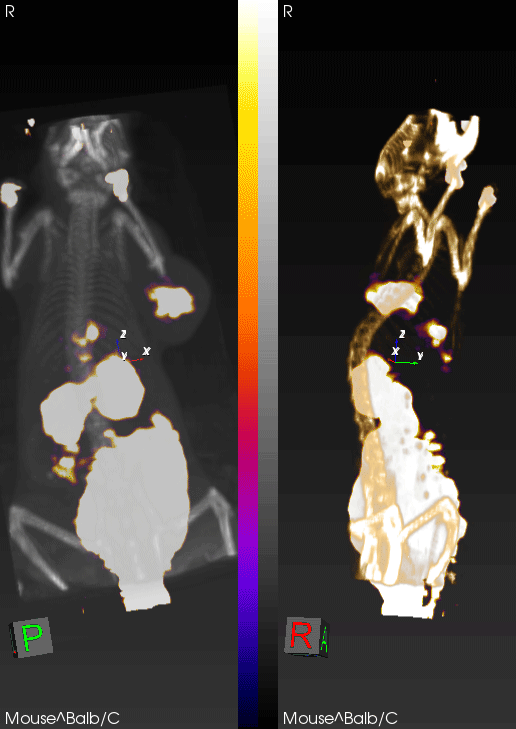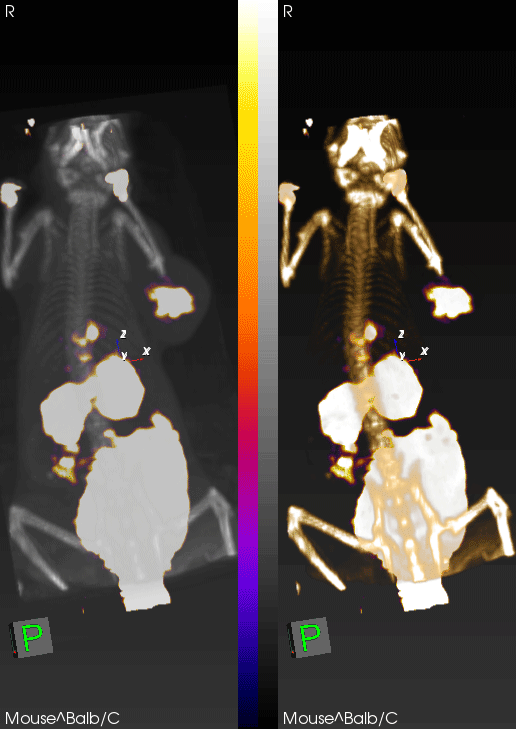
PET imaging can non-invasively detect changes in tumor levels of granzyme B, demonstrating that mice with high levels (green circles) respond to immunotherapy, whereas mice with low levels of granzyme B (red circles) do not respond.
